Therapeutic vaccination against HPV-related tumors: Study shows nanoparticles make difference
Researchers from the German Cancer Research Center (DKFZ) have collaborated with the SILVACX project...
View More
 English
English French
French Spanish
Spanish Hindi
Hindi Chinese
Chinese German
German Italian
Italian Japanese
Japanese Korean
Korean Russian
Russian Portuguese
Portuguese Arabic
Arabic Turkish
Turkish Dutch
Dutch
London, UK
Dear Colleagues,
We take great pleasure in inviting you to join us for the 4th World Forum on Infectious Diseases & Antimicrobial Resistance (IDAMR 2026), which will be held in London, UK, on June 25–26, 2026.
As one of the leading global congresses in the field, IDAMR 2026 is dedicated to advancing the practice, research, education, and innovation in the prevention, diagnosis, and treatment of infectious diseases, while addressing the growing threat of antimicrobial resistance.
Our participants include infectious disease specialists, microbiologists, clinicians, epidemiologists, virologists, bacteriologists, parasitologists, immunologists, pharmacists, nurses, public health experts, policymakers, industry leaders, and researchers who share a common mission of tackling the challenges posed by emerging infections and resistant pathogens.
This exciting event provides an outstanding platform to bring together the best minds from academia, industry, and healthcare to share groundbreaking findings, exchange knowledge, and foster collaborations that will shape the future of global health.
We look forward to your active participation in IDAMR 2026 this September in London. Please mark your calendars and join us in advancing the dialogue and solutions for infectious diseases and antimicrobial resistance.
Sincerely,
Organizing Committee
IDAMR 2026
World Forum on Infectious Diseases
1126 59 Ave East, V5X 1Y9, Vancouver BC, Canada


























Aspiring Scholars Directed Research Program (ASDRP)
USA
Zhejiang University School of Medicine
China
Affiliated Changzhou Children's Hospital
China
Xuanwu Hospital
China
Shanghai Donglei Brain Hospital
China
Hangzhou Chest Hospital
China
National Medical Institute
Poland
University of Electronic Science and Technology of China
China


















Basic definition of infection refers to the harm caused by a foreign particle entering the host body. Some are mild and few are life-threatening depending on the type of organism causing it. Starting from the cause, symptoms, detecting the organism, clinical studies, treatment, controlling it before spreading, preventing the infection, developing new methods and techniques for diagnosis to developing new drugs and treatment strategies, you can discuss at our Euro Infectious Diseases 2020 The global market for infectious disease treatments is a result of the growth in demand for new pharmaceutical treatments, vaccines development, and environmental products. In 2017, the global market for the treatment of infectious disease totaled $64.8 billion. The market should total $99 billion by 2022, growing at a CAGR of 8.9% from 2017 to 2022.
Market Analysis:The infectious diseases and global health market is a dynamic and expanding sector, primarily driven by the rising prevalence of infectious diseases, increasing antimicrobial resistance, and significant technological advancements. The market is broadly segmented into therapeutics (drugs and vaccines) and diagnostics. The therapeutics market, valued at around $124 billion to $243 billion, is dominated by viral infections and is projected to grow steadily, with North America holding the largest share due to its robust healthcare infrastructure and high R&D investment. Simultaneously, the infectious disease diagnostics market, valued at approximately $25 billion, is also seeing robust growth driven by the demand for rapid and accurate testing, particularly at the point of care. The overall market is shaped by global health priorities, including pandemic preparedness, and is increasingly leveraging digital health technologies like telemedicine and AI to improve disease surveillance, diagnosis, and patient care, with the global digital health market alone projected to reach over $1.5 trillion by 2032. For more details:
Similarly, in immunology, the focus on personalized medicine is leading to the development of tailored immunotherapies. However, both markets face challenges, including the high cost of advanced instruments and therapies, stringent regulatory processes, and a persistent shortage of skilled professionals, which can limit their full growth potential. Overall, these markets are poised for sustained growth, driven by an evolving landscape of scientific innovation and pressing global health needs.
nearly double to USD 18 billion by 2030 at an impressive ~12% CAGR, underlining the growing role of novel platforms in shaping the future of preventive care. Forward-looking analyses suggest that if next-generation delivery systems and therapeutic vaccines achieve wider clinical adoption, the overall market could surpass USD 150 billion by the mid-2030s. Numerically, this positions vaccines and immunizations as one of the most resilient sectors in healthcare, balancing predictable baseline demand with high-growth opportunities in innovation-driven subsegments.

The infectious diseases and antimicrobial resistance (AMR) market is growing as drug-resistant infections rise worldwide. The antibiotic resistance segment is valued at USD 9.3 billion in 2025 and projected to reach USD 12.1 billion by 2030 (CAGR 5.5%). Growth is fueled by increasing cases, public–private funding, and regulatory support. Asia-Pacific leads the market, South America shows the fastest growth, and pneumonia remains the largest disease segment.
The AMR diagnostics market, worth USD 4.6 billion in 2024, is expected to hit USD 6.7 billion by 2030 (CAGR 6.6%). Demand is rising for rapid, point-of-care tests, especially PCR-based technologies. North America currently dominates, but India and other emerging economies are expanding quickly, driven by the need for early detection and better antibiotic stewardship.
The AMR surveillance market is valued at USD 6.24 billion in 2025 and forecasted to reach USD 8.23 billion by 2030 (CAGR 5.7%). Growth is driven by molecular diagnostics, AI-powered analytics, and government programs. North America holds the largest share, while Asia-Pacific is the fastest-growing region. Hospitals remain the main users, with diagnostic labs gaining momentum.
Target Audience:Physicians | Internists | Clinicians | Infectious Disease Specialists | Microbiologists | Virologists | Bacteriologists | Parasitologist | Medical Students, Researchers, Residents Epidemiologists | Immunologists | Pharmacologists |Antimicrobial Stewardship Experts | Infection Control Officers | Healthcare Practitioners, Nurses | Allied Health Professionals, Physician Assistants | Pharmaceutical Researcher | Drug Discovery Scientists, Clinical Laboratory Scientists | Laboratory Technicians, Hospitals, Medical Colleges | Research Institutes, Pharmaceutical Industries | Biotechnology Companies, Drug Manufacturing Companies | Healthcare Workers and Druggist | Industries
valued at USD 3.5–4 billion in 2025, and expected to grow at a CAGR of 5% due to rising resistance in pathogens such as E. coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa. Federal funding has increased, with initiatives like CARB-X awarding over USD 360 million to early-stage AMR projects and BARDA investing more than USD 1 billion in advanced-stage antibiotic and diagnostic development. Rapid diagnostic tests for AMR are also gaining traction, with adoption projected to grow 2x by 2030.
Looking ahead, the U.S. infectious diseases and AMR market is poised for steady expansion, projected to reach USD 33–35 billion by 2030. Government incentives such as the FDA’s QIDP designation, which provides market exclusivity and priority review for new antibiotics, are encouraging innovation. However, the market faces hurdles: the average cost of antibiotic R&D exceeds USD 1.5 billion, while uptake remains slow due to stewardship programs and reimbursement challenges. Despite these barriers, strong federal support and industry investment position the U.S. as a global leader in infectious disease management, with increasing focus on next-generation antibiotics, vaccines, and precision diagnostics to combat AMR.
Market access is also moving: Europe has approved new agents (e.g., Emblaveo and Exblifep) for difficult Gram-negative infections, expanding the treatable addressable market while emphasizing antimicrobial stewardship frameworks at launch. Meanwhile, lawmakers debate transferable exclusivity vouchers (TEVs) as a pull incentive—industry sees them as catalytic, while public-health groups raise affordability and spillover concerns. Whichever design prevails, a predictable EU pull-incentive is the swing factor for late-stage pipeline investment and partnering decisions through 2030.
Workshops are typically interactive and hands-on sessions that allow attendees to learn practical skills or techniques, explore specific topics in more depth, and engage in discussions with other participants and experts in the field.
Contagious Diseases | COVID-19 | Emerging Infectious Diseases | Encephalitis | Gastroenteritis | Global Pandemic | Preparednes | Pediatric Infectious Diseases | Sepsis Diagnosis and Care | Vector-Borne Disease Control | Public Health and Epidemiology | Oral and Maxillofacial Infections | Genomics and Infectious diseases | Antibiotic Sensitivity Testing | HIV and Co-Infections | Hospital-Acquired Infections | Influenza Surveillance Systems | Malaria Elimination Strategies | Maternal-Fetal Infections | Microbiome and Immunity | Neglected Tropical Diseases | Next-Generation Antibiotics | Occupational Safety and Health | Point-of-Care Testing | Travel-Related Infections | Zoonotic Diseases |
The main aim behind the inclusion of workshops is to facilitate the transfer of knowledge through audience interactions. Workshops can be 3 to 6 hours in length, depending on the subject matter and relevance of the topics discussed. Organizers should select the workshop type and their preferred duration, in the workshop proposal. The following are the available possible workshop types: Regular workshop: 1 to 3 hours sessions with up to 5 presentations. Such workshops allow ample time for audience interactions. Skills building workshop: 3 to 6 hours sessions, organized to specifically build the capacity and knowledge of conference delegates. These workshops include only 1-3 presentations and focus a majority of their time on collaborative learning and skill development. Usually workshops would be panned with a number of presentations that will be followed by a discussion. The maximum limit of abstract submissions allowed for this type is 5. Workshops that combine 1-2 presentations with a panel discussion are still considered to be regular workshops. In these cases, we urge you to add the number of abstracts, and include the names and affiliations of the panellists.
e information such as - the value of the workshop, coherence between the presentation and related topic of the workshop, etc. Additionally, describe the format of your workshop in this section. The maximum number of words is 250.
2. Aim: Summarise the impact of the proposed workshop in a short messages of a maximum of 150 words.
Presentations: Select the number of presentations in this section. The maximum number of presentations for any workshop is 5 presentations.
3. Presenters: Select the number of presenters in this section. The presenters name, institute, and email address should be entered.
4. Social Media Statement: Provide us with a statement about your workshop using up to 120 characters that can be used for promotions on our various social media platforms. Be sure to use words or phrases that will capture attention and create a call to action, and include relevant hashtags.
Sheraton Heathrow Hotel Colnbrook By Pass, Harmondsworth, West Drayton, Middlesex, UB7 0HJ, United Kingdom
London holds status of the world's most powerful, most coveted, most influential, most visited, most expensive, most sustainable, most investment-friendly, and most popular-for-work city. Arts, commerce, education, entertainment, fashion, finance, healthcare, journalism, professional services, research and development, tourism, and transportation are all affected. In terms of economic performance, London is ranked 26th out of 300 big cities. It is one of the world's most important financial centres, with the fifth- or sixth-largest metropolitan area GDP. It is the most visited city in terms of international arrivals, and its airport system is the busiest in terms of passenger flow. It is the most popular investment location, with the most international shops of any city. After Paris, London has the second-highest number of ultra-wealthy persons in Europe in 2017.
are the heart of British political life. This site is celebrated for its stunning Gothic Revival architecture and the iconic chimes of the clock, which have become a symbol of London itself.
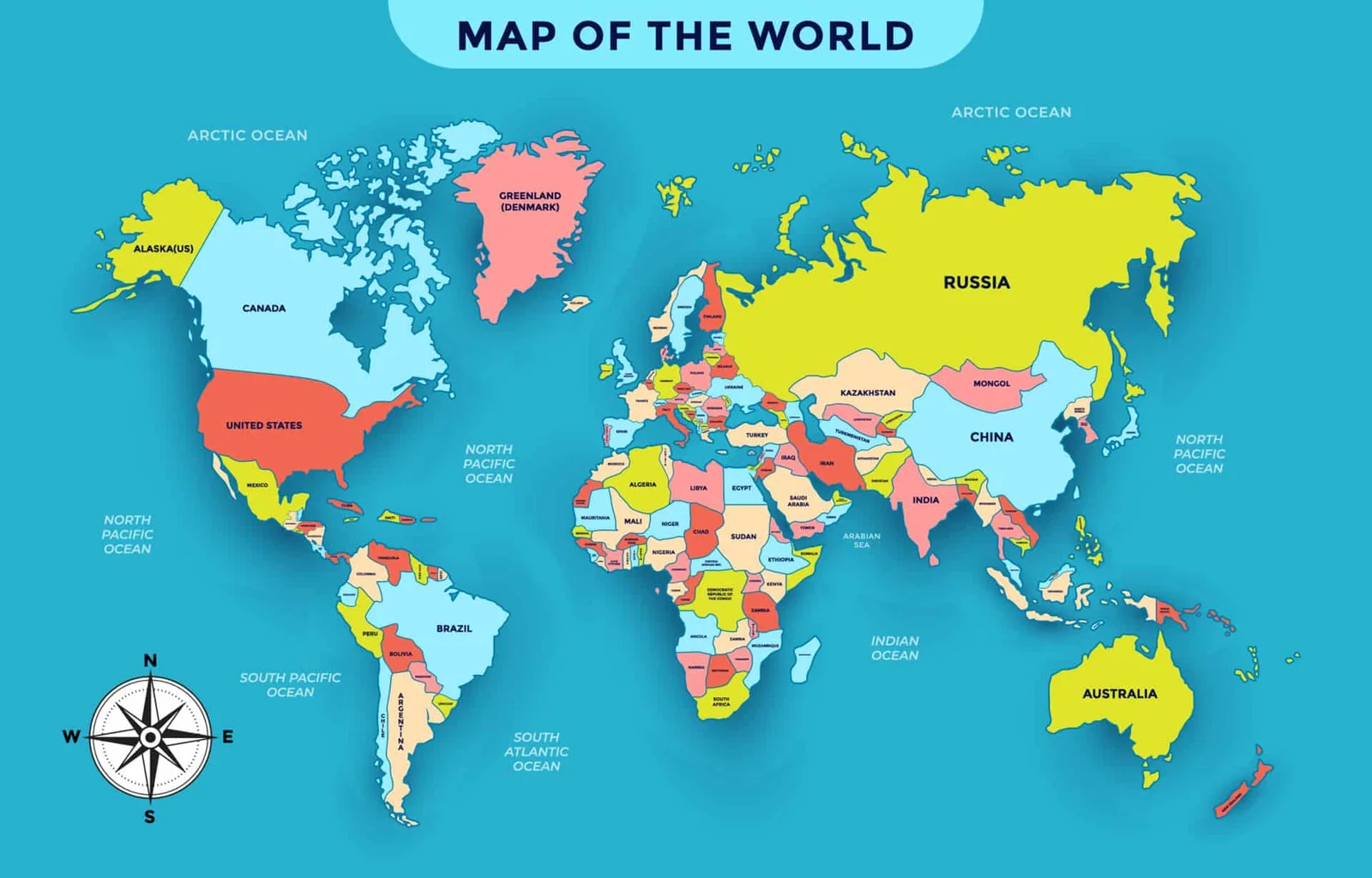
Clinicians & Infectious Disease Specialists 20%
Microbiologists & Virologists 15%
Pharmaceutical Researchers 10%
Healthcare Professionals 15%
Bacteriologists & Parasitologists 20%
Public Health Professionals & Faculty and Scientists 7%
Pathologists & Epidemiologists 5%
Biotechnology Companies & Drug Manufacturing Companies 8%


“Stay updated with the latest insights, highlights, and key takeaways from global conferences and business meetings.”
Researchers from the German Cancer Research Center (DKFZ) have collaborated with the SILVACX project...
View More
The global fight against infectious diseases faces two major challenges:
View More
A consortium of researchers with multidisciplinary skills, coordinated by INRAE and including the CNRS,...
View More
New microfluidic chip technologies are enabling faster, more accurate diagnostics for global healthcare...
View More
Scientists have leveraged AI to predict viral outbreaks and analyze large-scale epidemic trends...
View More
Biotech researchers have synthesized new antibiotic molecules showing potential against drug-resistant bacteria...
View More